Charder Medical Digital Hand Dynamometer MG4800 with Bluetooth
- Regular price
-
$299.00 USD - Regular price
-
- Sale price
-
$299.00 USD
Couldn't load pickup availability
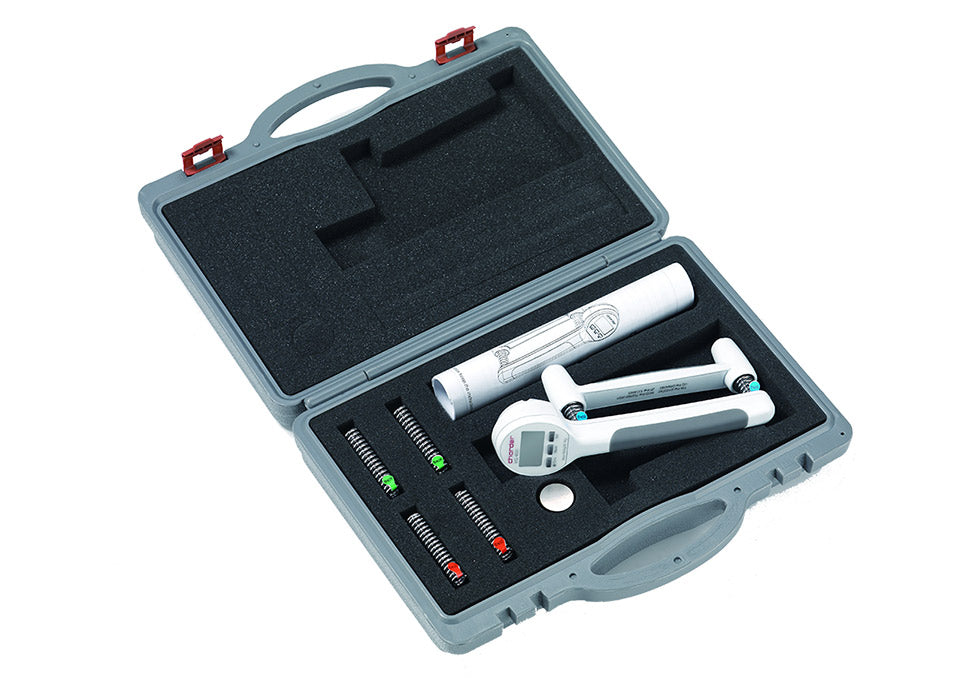
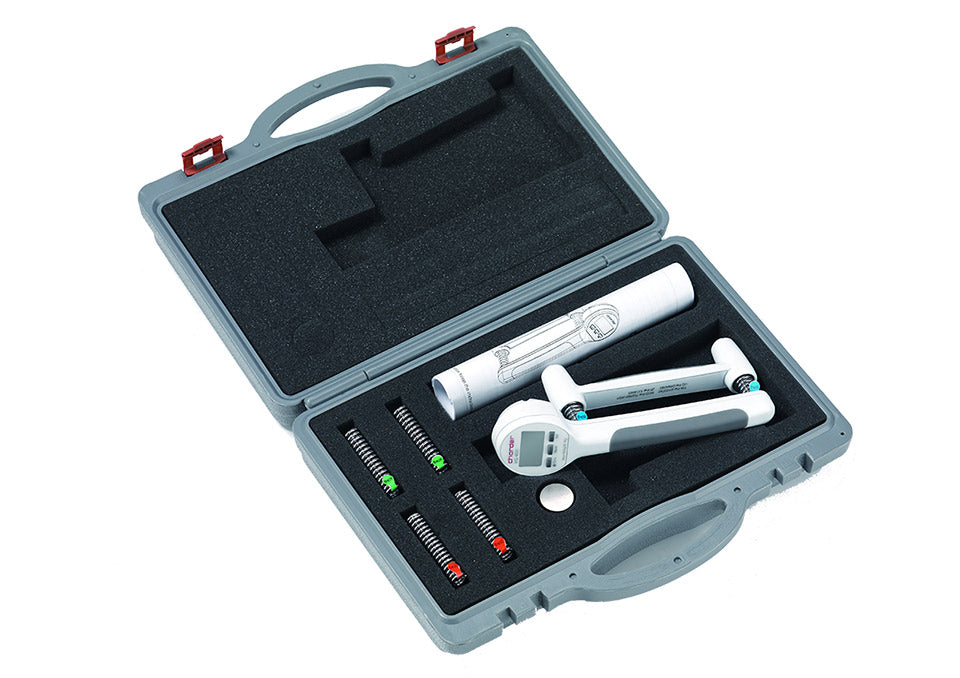
The Charder digital hand dynamometer with Bluetooth is an extremely versatile device for rehabilitation, with testing modes, and 3 sets of springs with different resistance levels. It is specifically engineered for arm rehabilitation and evaluation, making it an essential tool for occupational therapists, physical therapists, and athletic trainers.
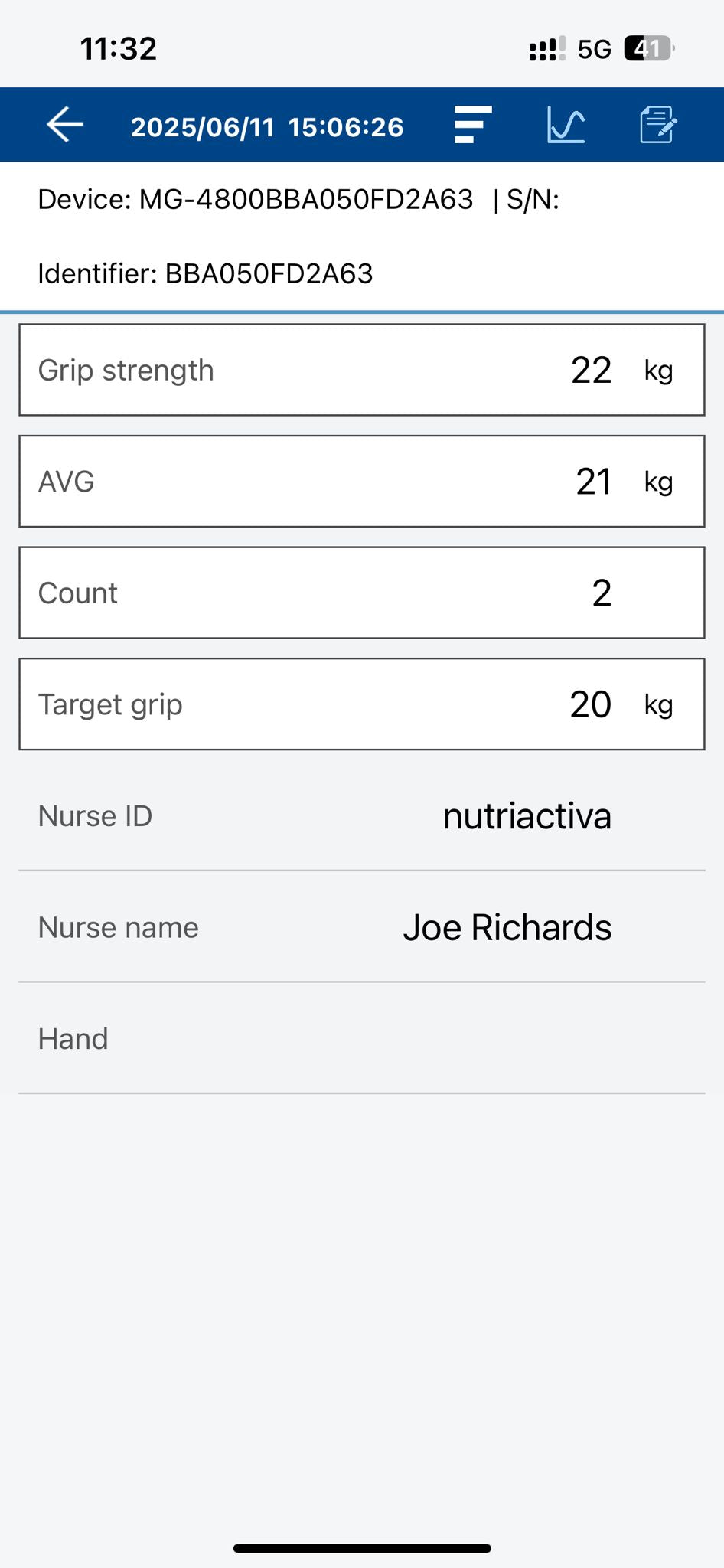
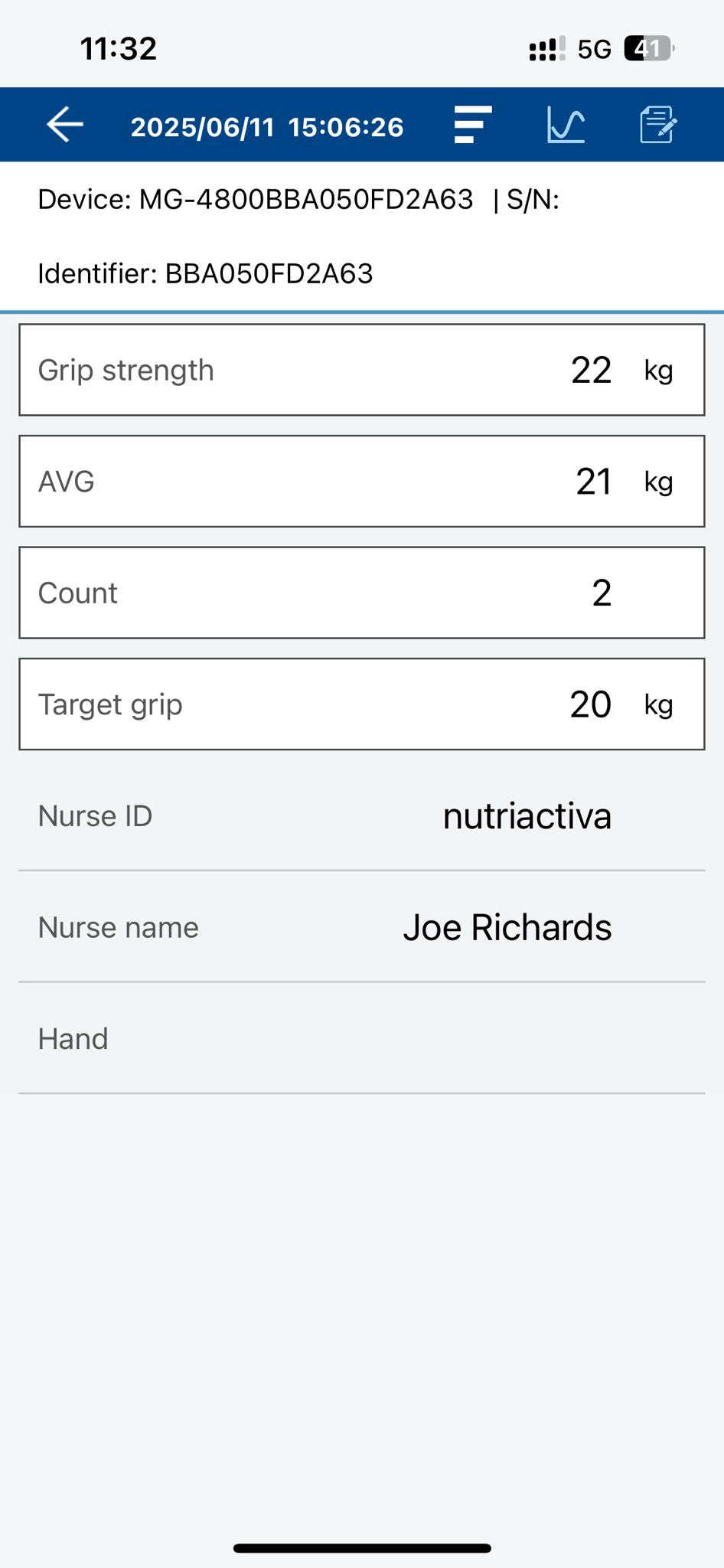
- THREE MODES Choose between maximum grip strength, average grip strength, and counting the number of repetitions that meet the selected minimum grip force.
- BLUETOOTH CONNECTION Connnets to the free Charder Connect app to store data for unlimited clients, for smartphones and tablets. Apple and Android
- MEDICAL GRADE It is both FDA approved for the USA and CE marked for the European Union.
- 3 RESISTANCE LEVELS FOR REHABILIATION The 20 kg / 40 kg / 80 kg springs allow the user to not only measure their grip strength, but to improve and train it as well.
- TRANSPORT CASE The solid case will keep the equiopment protected during transport and storage.
Measurements
- Handgrip strength
Specifications
- Capacity
- 80 kg (Blue spring)
- 40 kg (Green spring)
- 20 kg (Red spring)
- Accessories: Carry case, three standard spring settings
- Dimensions (case): 14 x 11 x 3.5 inches / 26 x 28 x 9 cm
- Weight (in case): 3 lbs / 1.32 kg
Shipping (USA)
Same day shipping from our USA warehouse on most orders placed by 1pm CST.
International Shipping

- Argentina
- Canada
- European Union
- Mexico
- United Kingdom
Customers from these countries who prefer to pay taxes upon import may request a refund before shipment.
For all other countries, import taxes will apply. You may contact us for a tax estimate.
Discounted DHL & Fedex rates are available to 200+ countries, usually arriving within 1 week.
Share






Charder Medical Digital Hand Dynamometer MG4800 with Bluetooth
- Regular price
-
$299.00 USD - Regular price
-
- Sale price
-
$299.00 USD
Shop With Confidence
-
30-Day Returns
-
1 Year Warranty